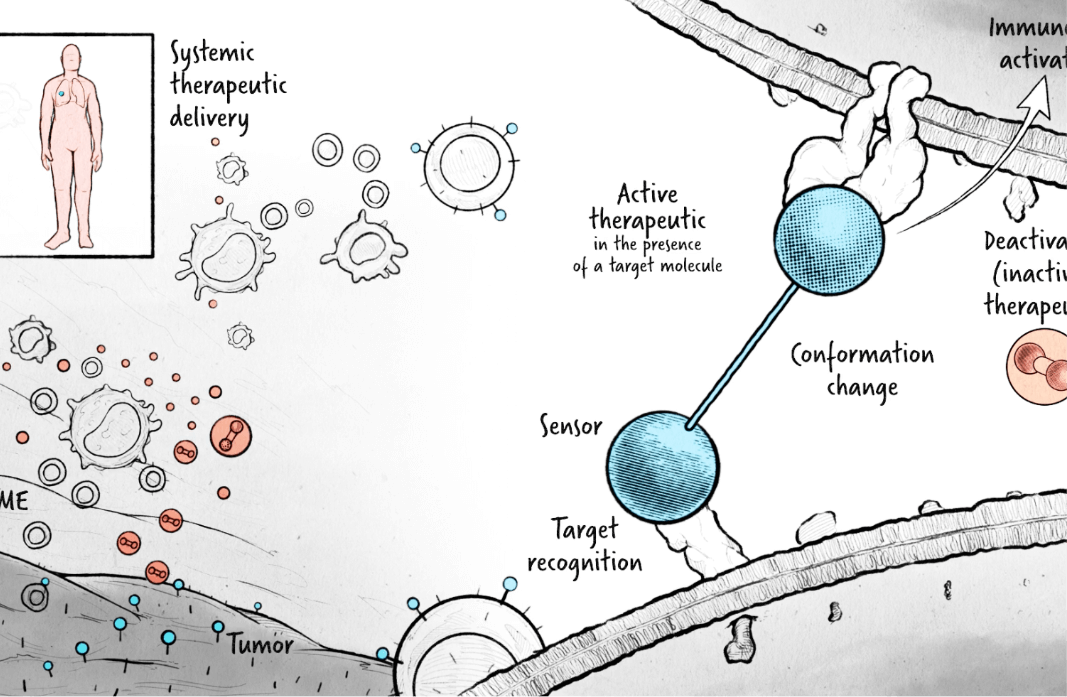
Systemic Delivery, Local Activity
Bonum Therapeutics uses a novel, antibody-based mechanism to create regulated and targeted, highly active, and less toxic medicines - two programs created with this platform are in preclinical development.
Our Science
We have created a new class of regulated protein therapeutics that deliver potent biological activity only where needed. Our molecules contain a sensor domain that, when bound to its target, activates the molecule’s therapeutic component. Our sensor domains can be engineered to target any entity that an antibody can bind, including peptides, proteins, and metabolites.
Our current pipeline of regulated cytokines with antibody controlled activity is focused on the treatment of cancer. We are also interested in potential applications in autoimmune disease, metabolic disorders, and pain management – all areas where safer and more effective treatments are needed
Antibody
Controlled therapeutics
Our therapeutics allow for regulated, antibody controlled activity by remaining inactive until bound to their target. Unlike protease-dependent approaches, this regulation mechanism is reversible – the therapeutic reverts to an inactive form if it disengages its target.
Currently, we are developing antibody controlled cytokine-based therapeutics designed to enhance the anti-tumor immune response. Our programs include approaches that target immune cells as well as strategies directed towards additional cell types within the tumor microenvironment.
See Our Programs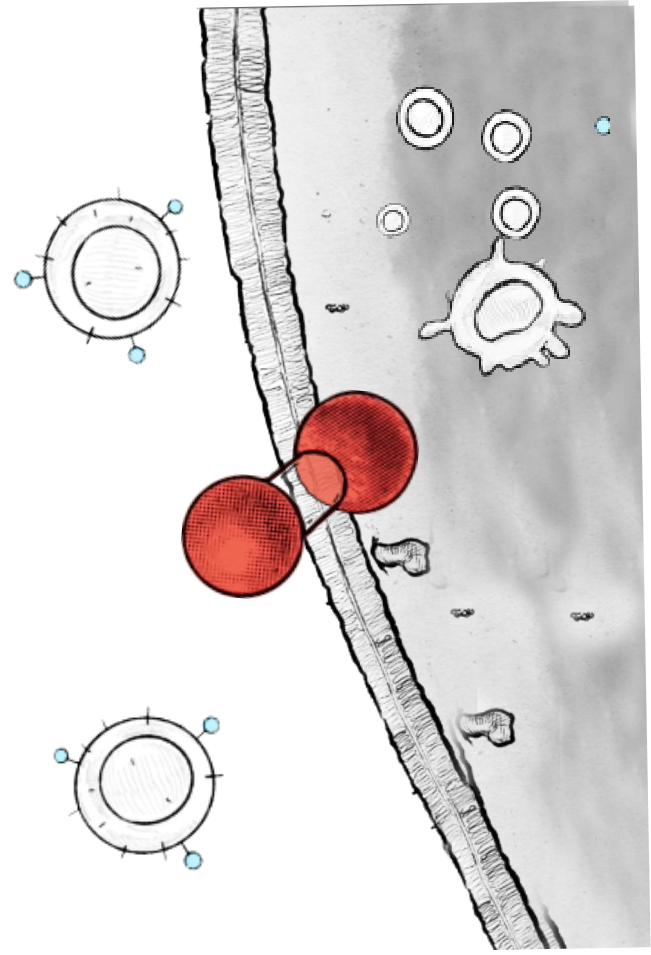
Our Experienced Team
The Bonum Therapeutics team has deep expertise in innovation and drug development.
About Us
Join Us
Bonum Therapeutics is seeking scientists with experience across drug discovery and development to join us in our quest for safer and more effective therapeutics.
Careers